Workers, primarily those in the health care profession, involved in treating and caring for individuals injured or sick, can be exposed to biological liquids capable of transmitting disease. These diseases, which may be caused by a variety of microorganisms, can pose significant risks to life and health. This is especially true of blood-borne viruses that cause hepatitis [Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV)] and acquired immune deficiency syndrome (AIDS) [Human Immunodeficiency Virus (HIV)]. Since engineering controls cannot eliminate all possible exposures, attention is placed on reducing the potential of direct skin contact through the use of protective clothing that resists penetration. This test method was developed for ranking the synthetic blood penetration resistance performance of medical face masks in a manner representing actual use as might occur when the face mask is contacted by a high velocity stream of blood from a punctured wound.
The test method is intended to evaluate the protection of the health care provider’s face from exposure to blood and body fluids. It is used to evaluate the resistance of medical face masks to penetration by synthetic blood under high-velocity liquid contact with the medical face mask surface of a fixed volume over a relatively short period of time (0 s to 2,5 s). Medical face mask “pass/fail” determinations are based on visual detection of synthetic blood penetration.
NOTE 1 Medical face masks are intended to resist liquid penetration from the splatter or splashing of blood, body fluids, and other potentially infectious materials. Many factors can affect the wetting and penetration characteristics of body fluids, such as: surface tension; viscosity; and polarity of the fluid, as well as the structure and relative hydrophilicity or hydrophobicity of the materials. The surface tension range for blood and body fluids (excluding saliva) is approximately 0,042 N/m to 0,060 N/m[1]. To help simulate the wetting characteristics of blood and body fluids, the surface tension of the synthetic blood is adjusted to approximate the lower end of this surface tension range. The resulting surface tension of the synthetic blood is (0,042 ± 0,002) N/m.
NOTE 2 During a medical procedure, a blood vessel can be punctured resulting in a high-velocity stream of blood impacting a protective medical face mask. The impact velocity depends on several factors, the most important being the blood pressure of the patient. A second factor is the distance from the puncture. The velocity of larger punctures drops because the pressure in the blood vessel drops quickly. Because only small punctures cause high-velocity streams, large punctures were not used to model the range of blood-splatter velocities considered in this test. Furthermore, this test method is based on the assumption that the medical face mask will be in close proximity to the puncture area. This test method is therefore based on the impact velocity of a stream of fluid that corresponds to the target blood pressure.
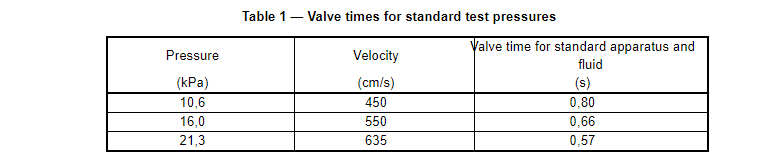
NOTE 3 The mean human blood pressure generally varies over a range of about 10,6 kPa to 16,0 kPa (80 mm Hg to 120 mm Hg)[2]. In this test method, medical face masks are tested at stream velocities corresponding to 10,6 kPa, 16,0 kPa, and 21,3 kPa (80 mm Hg, 120 mm Hg, and 160 mm Hg, respectively). This test method permits the use of other non-standard test pressures, stream velocities, fluid volumes, and specimen orientations for evaluating medical face mask penetration resistance consistent with specific applications.
This International Standard does not apply to all forms or conditions of blood-borne pathogen exposure. Users of the test method should review modes for face exposure and assess the appropriateness of this test method for their specific application.
This International Standard primarily addresses the performance of materials or certain material constructions used in medical face masks. This test method does not address the performance of the medical face mask's design, construction, interfaces or other factors which may affect the overall protection offered by the medical face mask and its operation (such as filtration efficiency and pressure drop).
This test method does not address breathability of the medical face mask materials or any other properties affecting the ease of breathing through the medical face mask. This test method evaluates medical face masks as an item of protective clothing. This test method does not evaluate the performance of medical face masks as protection against contamination via airborne exposure pathways or in the prevention of the penetration of aerosolized body fluids deposited on the medical face mask.
NOTE 4 Users of this test method should realize that certain tradeoffs exist between improved resistance of medical face masks to penetration by synthetic blood and in pressure drop across mask materials which is an indicator of the breathability of the face mask. In general, increasing synthetic blood penetration resistance for medical face masks results in increasing pressure drop or reduced breathability for medical face masks of the same design and fit of the individual wearer.
NOTE 5 This test method evaluates medical face masks as an item of protective clothing and does not evaluate medical face masks as respirators. If respiratory protection for the wearer is needed, an approved respirator should be used. This test method can be used to evaluate the resistance of a respirator to penetration by synthetic blood, if warranted.
Testing Procedure
1、Preparation and cleaning of test apparatus
NOTE 1 An alternative test set-up procedure is provided in 7.3 that utilizes a targeting plate to ensure a more accurate and uniform velocity of fluid to the specimen mask.
Prepare and clean the test apparatus using the following steps.
a) Install a clean 12,7 mm long canula with an inside diameter of 0,84 mm on the front of the pneumatic-controlled valve.
b) Fill the reservoir with new synthetic blood (approximately 1 l).
c) Set the valve time corresponding to the blood pressure being assessed in accordance with Table 1. If non-standard pressures, fluid volumes (2 ml) or canula sizes (0,084 cm ID) are employed, the valve time should be calculated using Equations (C.4) and (C.7) in Annex C.
NOTE 2 For the purposes of this test method, as a minimum three different sets of specimens at stream velocities corresponding to blood pressures of 10,6 kPa, 16,0 kPa, and 21,3 kPa are evaluated.
d) Adjust the reservoir pressure as needed to achieve a flow of 2 ml for the selected valve time.
e) Verify the amount of synthetic blood delivered to be 2 ml by conducting trials into a graduated cylinder.
Alternatively, the volume of synthetic blood can be measured by determining the mass using a balance. For the standard fluid, with a specific gravity of 1,005, the 2 ml of fluid would weigh (2,010 ± 0,040) g.
f) After every 16 specimens, ensure that the text apparatus is delivering 2 ml of synthetic blood by following the method calibration steps as directed in 7.1 d) and 7.1 e).
g) If the canula is left unused for 1 h or more after synthetic blood has passed through it during testing, replace it with a clean canula and clean the used canula.
h) Clean the canula by immersing in isopropanol for 24 h and rinsing with distilled water.
i) Following testing, clean the system lines and the reservoir with distilled water. Do not use isopropanol or other solvents on the valve or system lines as the valve may be damaged.
2、Test procedure
Use the following steps to evaluate medical face masks.
a) Conduct all testing in an environment having a temperature of (21 ± 5) °C and a relative humidity of (85 ± 10) %.
b) Place a small droplet (approximately 0,1 ml) of the synthetic blood on the normal inside surface of an extra medical face mask. The droplet shall be easily visible to ensure that any droplet that penetrates the material will be seen. If not, use talcum powder on the normal inside surface of the medical face mask to enhance droplet visibility.
c) Remove a specimen from the conditioning chamber. Mount the specimen on the specimen-holding fixture and position the specimen for impact of the synthetic blood to occur in the target area.
If the face mask contains pleats, spread the pleats out when mounting the face mask onto the test fixture to present a single layer of material as the target area. Use the centre of the specimen as the target area.
Position the end of the pneumatic-controlled valve at a distance of (300 ± 10) mm from the target area from the specimen.
d) Squirt the synthetic blood onto the specimen medical face mask. Ensure that the synthetic blood hits the target area of the medical face mask. Conduct the test within 60 s after removal from conditioning chamber.
e) Inspect the viewing side of the specimen for synthetic blood (10 ± 1) s after squirting the synthetic blood against the target area. Note whether any synthetic blood or other evidence of wetness, or both, appears on the viewing side of the specimen using suitable lighting.
Use a cotton absorbent swab or similar item to lightly daub the target area if there is any doubt regarding the visible penetration of the synthetic blood.
f) Test the remaining specimens.
3、Alternative test set-up using a targeting plate
The following procedure improves the accuracy of the velocity of the stream hitting the target mask. Once the valve opens, the pressure of the fluid at the tip drops as frictional losses build as the fluid flows through the tubing, valve and canula. The net result is that the pressure of the initial portion of the stream can be two to three times the target pressure. This procedure blocks this high-pressure stream and allows only the fluid travelling at the target velocity to hit the mask.
a) Set the valve time to 0,5 s.
b) Collect and weigh the amount of fluid delivered from the nozzle.
c) Set the valve time to 1,5 s.
d) Collect and weigh the amount of fluid delivered from the nozzle.
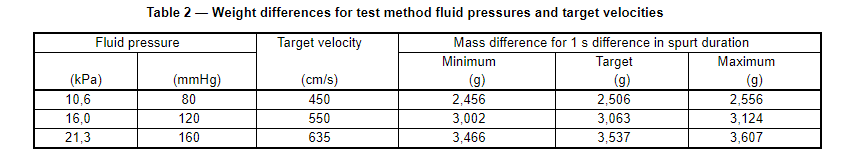
e) Calculate the difference in mass of the two spurts. For a test fluid with a specific gravity of 1,005, Table 2 gives the target difference in mass plus lower and upper limits for a velocity range within 2 % of the target. See Annex B to determine the target mass differences for other velocities, canula sizes or fluids with other specific gravities.
f) Adjust the reservoir pressure as necessary and repeat steps 7.3 a) through 7.3 e) until the mass difference is within the target range.
g) Once the reservoir pressure has been set, do not change the relative height of the reservoir and nozzle.
h) The targeting plate should be placed approximately 1 cm away from the mask and be located such that the fluid passing through the hole in the targeting plate hits within 0,6 cm of the centre of the hole in the specimen holding form.
i) Adjust the aim of the valve assembly such that the steady-state portion of the stream passes cleanly through the targeting hole. The initial portion of the stream should hit above the hole.
j) Set the valve time to 0,5 s.
k) Collect and weigh the amount of fluid passing through the targeting hole.
l) Set the valve time to 1,5 s.
m) Collect and weigh the amount of fluid passing through the targeting hole.
n) The difference in mass between the 0,5 s and 1,5 s deliveries through the targeting plate hole should be within mml_m1 of the difference in mass from the nozzle [7.3 f)].
o) If the differential mass through the hole is less than 95 % of the mass difference exiting the nozzle, check the aim of the stream to make sure it is passing cleanly through the targeting hole.
p) If the mass differential is more than 102 % of the mass difference exiting the nozzle, repeat the collecting and weighing process in 7.3 a) to 7.3 f).
q) Adjust the timer setting until 2 ml of fluid passes through the hole for three spurts in a row. For a test fluid with a density of 1,005 g/cm3, the output should weigh 2,01 g.
r) Record the timer setting to use as the starting point for subsequent testing.