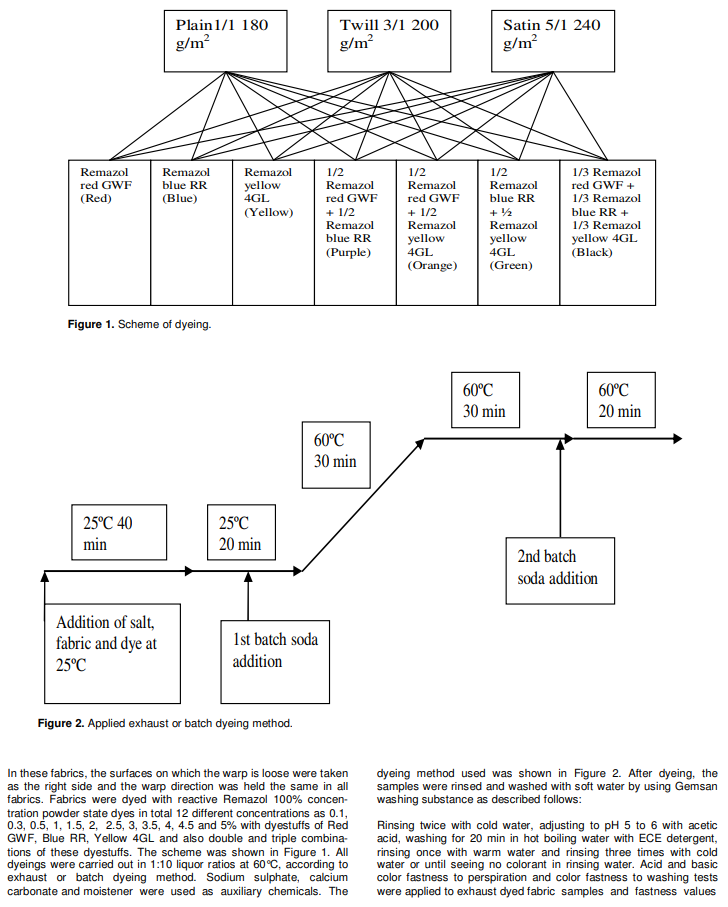
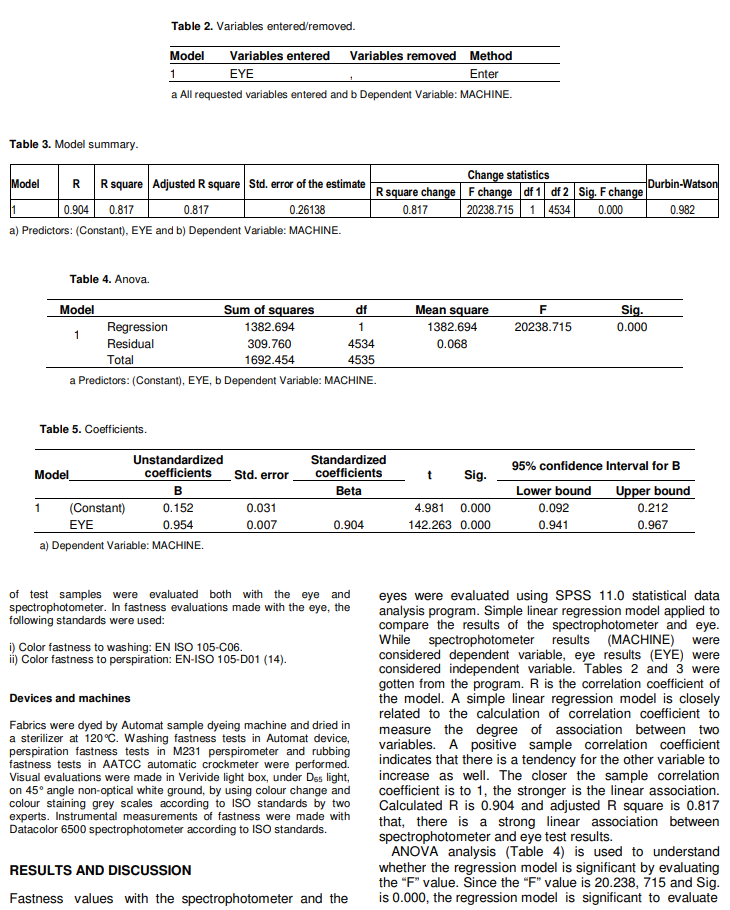
The chemical reaction between reactive dyestuffs and a cellulosic fibre takes place in the presence of a base and reactive dyestuffs are covalently bonded to cellulosic fibre according to the following two sided reaction: Cel ?OH +Cl ? dye _ mol ? Cel ?O ? dye _ mol + salt (1) The covalent bond thus formed provides very good fastness properties and is much stronger than the weak hydrogen bonds of direct dyestuffs with cellulose fibres.Reactive dyestuffs react in a similar way with amino groups. The reaction with amino groups also provides their use on dyeing protein fibres and polyamide fibres (Tarakcioglu, 1979; Rivlin, 1992). Reactive dyes are applied to cellulosic fibers by a variety of processes including exhaust or batch dyeing method, pad-batch,pad-steam, pad-bake, print-steam, and print-bake.Currently, padding and printing processes account for about 30% of the market, the residual, most popular application method being “exhaust” dyeing. The reason for the current popularity of the latter method lies in the short-run, high fashion nature of the textile industry,which also requires that coloration should be delayed to be piece goods or even garment stage.
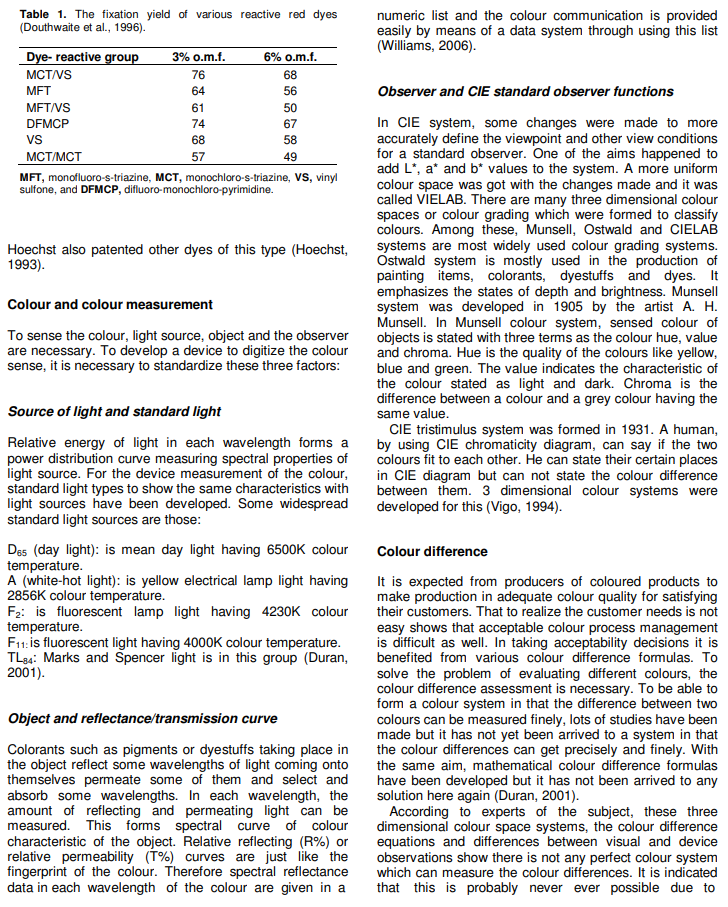
Dye is lost to dye-house effluent for a number of reasons, but reactive dye hydrolysis during the application is the most important one. An analysis of this situation was carried out and published in Table 1 comparing dye fiber covalent bonding efficiencies versus type of reactive group; “fixation” or covalent-bonding efficiency by X-ray fluorescence of bound sulfur associated with the sulfonated chromophore. Table 1 indicates that in medium shades up to 30% of the dye applied ends up in the effluent, whereas in full depths up to 50% of the dye may be washed away. Given that reactive dyes are highly water soluble, it is difficult to remove from dye-house effluent at cellulose fiber dyeing using reactive dyes (Lewis, 2008). It is well known that increasing the number of reactive groups attached to the dye molecule increases fixation yields and in consequence, there are many bi-functional dyes on the market. If tri-functional dyes could be economically manufactured then it might be expected that improved cotton reactive dyeing efficiencies would result (Lewis, 2008).
As early as 1975 Hoechst launched the tri-functional dye, Remazol Red SBB which contained a monochloro-striazine group plus two divinylsulfone groups linked through an aliphatic amine to the triazine residue;
Conclusions:
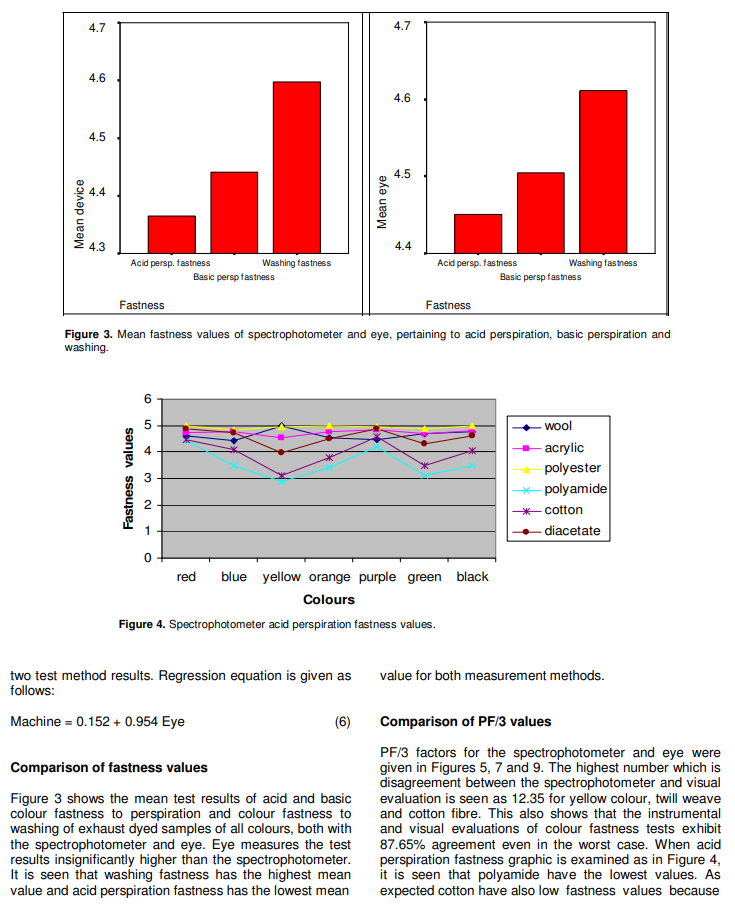
In this study it was seen that light shades have lower deviations than medium shades. The highest PF/3 factor was 12.35 within all data sets and this means that consistency changes between 87.65 and 100%.
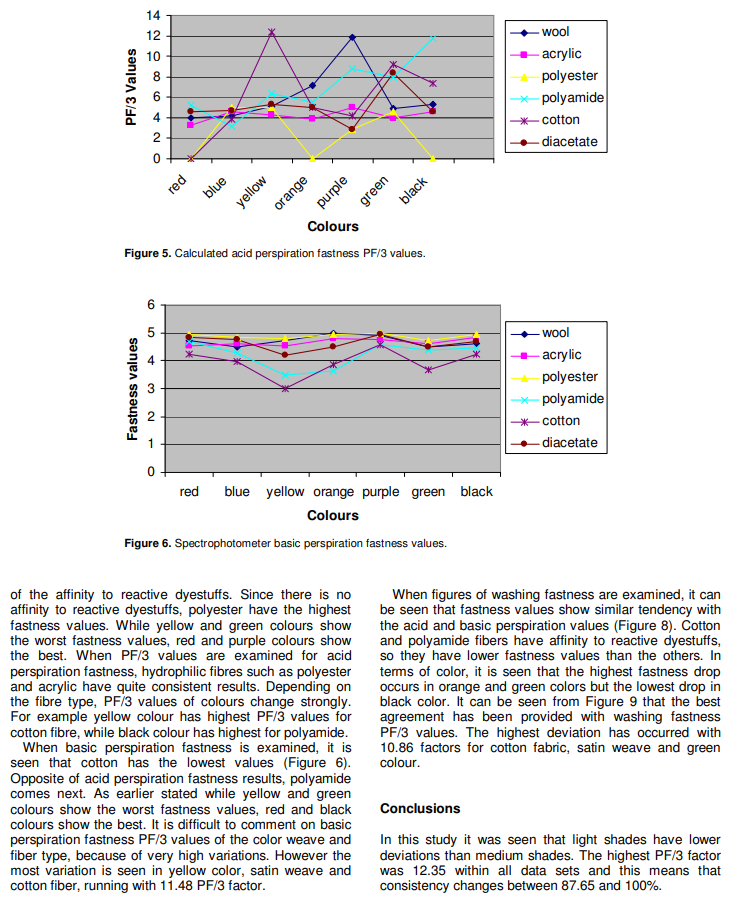
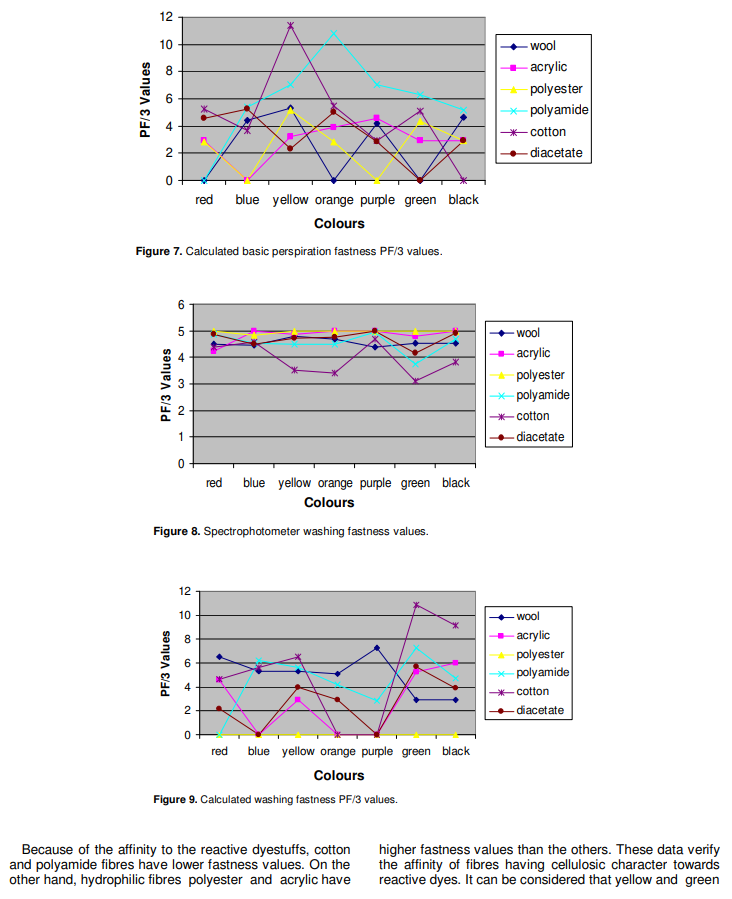
Because of the affinity to the reactive dyestuffs, cotton and polyamide fibres have lower fastness values. On the other hand, hydrophilic fibres polyester and acrylic have higher fastness values than the others. These data verify the affinity of fibres having cellulosic character towards reactive dyes. It can be considered that yellow and green Colours have the lowest fastness values but the highest PF/3 values. According to the data, acid fastness values of double and triple mixed dye coloured fabrics are lower than that of single dye coloured fabrics.
Weave type has no significant effect on the fastness values.